What You Should Know About CSV in Pharma
Por um escritor misterioso
Descrição
Learn more about computer system validation, which is required by the FDA and other global regulatory bodies for drug and medical device manufacturers.
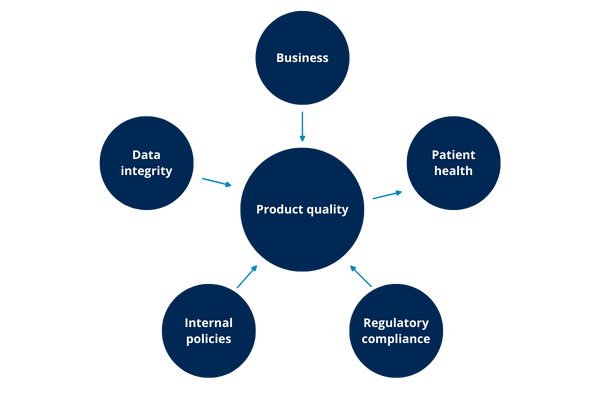
A Complete Guide to Computer System Validation (CSV): What is it and why do we need it?
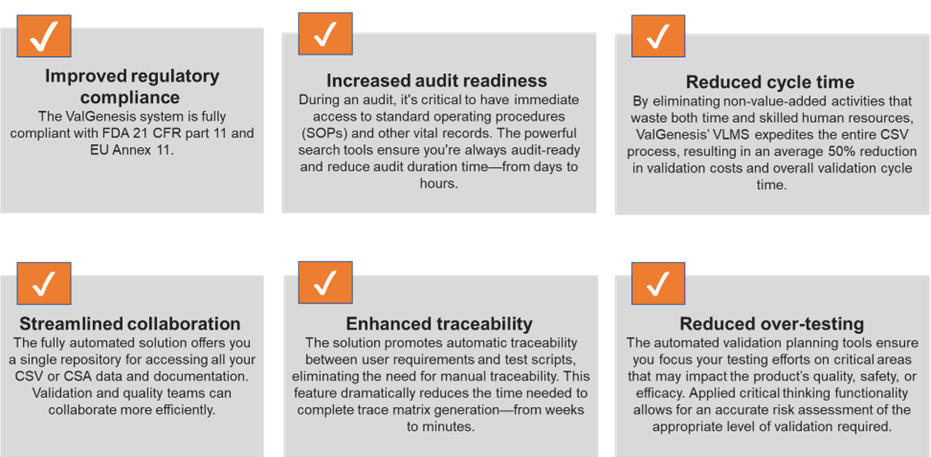
6 Common Challenges of Paper-Based computer system validation (CSV) and How to Digitize Them - Astrix

Software Validation: Here's How We Do It - Apprentice

9 of the Best Online CSV Resources for Pharma Validation Professionals

What You Should Know About CSV in Pharma
A Complete Guide to Computer System Validation (CSV): What is it and why do we need it?

What is CSV, Its Need, Purpose & Benefits in pharma Industry. – Be Innov@tive
Download Program, Are you compliant with FDA requirements for CSV, Data Integrity & Process Validation? Today's regulators are applying more

CSV, Data Integrity & Process Validation Virtual Summit, 2021

PDF) Computer system validation in the perspective of the pharmaceutical industry
de
por adulto (o preço varia de acordo com o tamanho do grupo)



format(webp))


